Incubation
Fertile Eggs
The nucleus of the female cell is a small white or light-coloured speck about the size of a pinhead that is located on the top side of the yolk. Here the microscopic male sperm cell finds lodgement and the cells are united to form the embryo. A fertilised egg is characterised by a white ring 3-4 millimetres in size on the yolk surface (germ cell), whereas an infertile egg is characterised by a single white speck of about 2-3 mm diameter.

Fertile eggs should be stored point down
Fertile Egg Storage
Fertile eggs should be clean and dry and stored between 12-15°C at a relative humidity of 75% with the small end down.
Eggs should be turned by 90 degrees at least once to twice daily. Optimal hatchability is achieved in fresh eggs less than 10 days old, but reasonable hatchability can be obtained in eggs up to 14 days of age. Fertile eggs should maintain a relatively constant weight with minimal weight loss during storage. Temperatures above 25°C can initiate cellular replication of the germ cell on the yolk of the fertile egg and will increase embryonic mortality and reduce hatchability. Temperatures below 10°C can inactivate the germ cell.
Principles of Artificial Incubation of Fertile Eggs
The four main essentials of incubation of good quality fertile eggs are:
- Correct and even temperature controlled by a thermometer or thermocouple
- Correct humidity controlled by ventilation rate and water application
- Correct oxygen and carbon dioxide concentrations controlled by ventilation
- Turning of the fertile eggs by approximately 90 degrees several times per day by manual or automatic means.
These parameters can be easily achieved and maintained if the incubator manufacturer’s operating instructions are carefully adhered to.
Incubation Temperature Range and Variation
The temperature requirements for incubation are described in Table 1 (below) and most incubators have a temperature variation of 0.2-0.4°C for effective incubation and subsequently a high hatchability rate.
Embryo tolerance to temperatures more than 1°C above or below the recommended temperature (Table 1) is low, and temperatures outside this range will result is significant embryonic mortality. Embryos are much more susceptible to temperature variation in the early and late phases of incubation.
Incubation Relative Humidity Range and Variation
The maintenance of consistent relative humidity is more difficult during incubation and can only be constantly maintained by ventilation rate, using adjustable ventilation apertures and by surface water and water sprays during incubation. The tolerance of the embryo to different ranges of humidity are greater than temperature, but there are negative consequences observed with humidity below 40% and above 90%. Good hatchability is achieved when relative humidity is maintained at approximately 50-65% until the last 3 days of incubation, at which point it should be increased to between 70-90%.

Fertile eggs are rotated in an incubator
Ventilation and Carbon Dioxide/Oxygen Concentration
Embryonic growth is optimised at an air concentration of carbon dioxide of 0.4% and embryonic growth is depressed and mortality increased with carbon dioxide concentrations above 1%. The normal atmosphere contains 21% oxygen and 0.04% carbon dioxide. The hatched chick is most susceptible to oxygen deviation (compared to the pipped chick and the embryo in the intact egg), which implies that ventilation rate and carbon dioxide concentration is most critical in the late phase of incubation.
Egg Turning and Rotation
Egg rotation or turning is required to ensure that the embryo developing on the yolk does not adhere to the shell membrane. This phenomenon of adherence to the shell membrane commonly occurs during fertile egg storage and during early incubation (generally the first week). The turning process allows the embryo to revolve and slide in the inner white and provides access to additional nutrients for embryonic development. Egg turning should be undertaken 3-6 times per day and an uneven number of rotations are better so that the eggs are not in the same position for a longer period. Most incubators rotate the eggs by approximately 90 degrees.
Table 1. Incubation Period and Operating Conditions for Fertile Eggs of Domestic Birds in Forced Draught Incubators
| Fowl | Turkey | Duck | Muscovy Duck |
Goose | Pheasant | Guinea Fowl |
Quail | |
| Incubation Period days | 21 | 28 | 28 | 35 | 28 | 23-28 | 28 | 23-24 |
| Incubation Temperature (°C) |
37.6 | 37.4 | 37.5 | 37.5 | 37.4 | 37.6 | 37.6 | 37.6 |
| Wet Bulb Temperature (°C) |
29.4-30.5 | 28.3-29.4 | 28.8-30 | 28.8-30 | 30-31.1 | 30-31.1 | 28.3-29.4 | 28.8-30 |
| Relative Humidity (%) | 56-62 | 51-56 | 53-60 | 53-60 | 60-65 | 60-65 | 51-56 | 53-60 |
| No of Daily Turning | 18 | 25 | 25 | 31 | 25 | 21 | 25 | 21 |
| Incubation Temperature last 3 days (°C) |
37.4 | 37.2 | 37.3 | 37.3 | 37.2 | 37.4 | 37.4 | 37.4 |
| Wet Bulb Temperature last 3 Days (°C) |
32.2-34.4 | 32.2-34.4 | 32.2-34.4 | 32.2-34.4 | 32.2-34.4 | 33.3-35 | 32.2-34.4 | 32.2-34.4 |
| Relative Humidity last 3 days (%) | 70-83 | 70-83 | 70-83 | 70-83 | 70-83 | 76-90 | 70-83 | 70-83 |
For still air incubators add approximately 1°C to the operating temperatures recommended in table 1. This is because the thermometer in still air incubators is normally located at the top of the incubator and there is a marked temperature gradient from the top of the incubator to the bottom.
Candling of Incubated Fertile Eggs
After 5-8 days of incubation, the eggs should be examined using a candling light to examine the embryo for blood vessel development (‘spider web-like’) and a dark spot. Infertile eggs are obviously clear with no evidence of blood and early embryonic death is noted by the presence of a blood ring surrounding the yolk. Infertile and early dead embryos are removed at this stage. Candling can also be undertaken at 18 days of age, where the embryo is clearly visible with a distinct dividing line between the embryo and the air cell.
In large commercial incubators, candling is not normally undertaken and there is a high reliance on fertility and egg hygiene to maintain viable embryos
Chicken Embryo Development
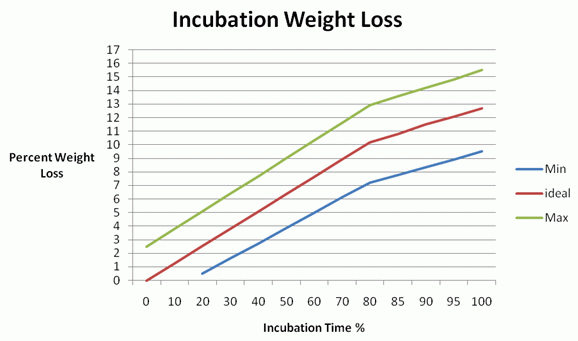
Figure 1. Percent weight loss during incubation period with ideal weight loss and tolerances illustrated with maximum and minimum values.
Weight Loss during Incubation
Eggs that contain a growing embryo will progressively dry-out throughout incubation. This results in an overall weight loss of the egg and this progressive weight loss can be objectively monitored to improve incubation success. The data below (Figure 1.) is a good guide. Weight loss patterns should be monitored and the ideal weight loss (13%) achieved if possible.
Main Incubation Failures
Modern electronic technology has been developed to manage variation in temperature and humidity during incubation and these parameters are monitored constantly and recorded along with the incubation settings. This enables the relationship between the parameter, settings and hatchability to be analysed in relation to embryonic mortality. Analysis of dead embryos in the shell is also a valuable problem solving tool to help refine incubation practices.

- Early hatched, weak chicks, unhealed navels, unabsorbed yolk sacs, crooked toes, crossed beaks and a high proportion late dead in the shell, are indications that the incubation temperature was too high.
- Late hatching of large soft chicks which are slow starting, and chicks with wrynecks, are characteristic of incubation temperatures that were too low.
- Large numbers of unhatched, unpipped chicks, live trapped embryos and large chicks coated with albumen are indications that the incubation humidity was too high.
- Small weak chicks with large air cells, exhausted chicks in shells that have been chipped most of the way around, and chicks glued to the shell are indications that the relative humidity was too low.


